Liquid water is held together by hydrogen bonds. (Liquid water has fewer hydrogen bonds than ice.) Oils and fats not have any polar part and so for them to dissolve in water they would have to break some of water’s hydrogen bonds. Water will not do this so the oil is forced to stay separate from the water.

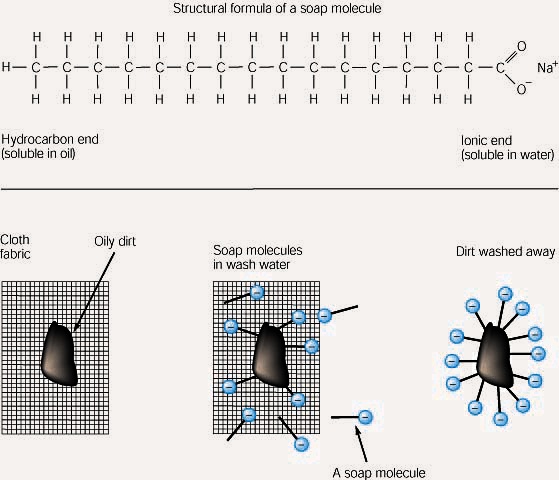
Soap cleans oil and grease because one end of the soap molecule is polar and so is soluble in water, and the other end is non-polar and so similar to oil and grease. The soap molecules surround the grease leaving the water-soluble parts on the outside so the water can help wash the grease away. Thus, the soap molecule provides a link between two substances that would otherwise be immiscible.
Please Watch animation 10.2 on polar and non-polar solutions..