 |
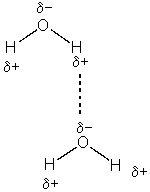
Water can form crystals. How? The H2O molecule is stable. The answer lies in the polarity of the bonds. Oxygen and hydrogen do not share the electrons equally. The oxygen has a greater pull on the shared electrons than the hydrogen. The extra electron density creates a partial negative charge on the oxygen and a this leaves a partial positive charge on the hydrogen. In the picture below, please notice that the solid bonds are the covalent bonds and the dashed bond is the bond between molecules. This particular intermolecular bond is called a hydrogen bond. A hydrogen bond is much weaker than a covalent or ionic bond but it is the strongest of the intermolecular bonds.

When water is a solid, hydrogen bonds form to make a regular pattern of water molecules. We can see, however it is much easier to break these hydrogen bonds than the covalent bonds in the diamond crystal. Even the heat above 0°C can break enough of the the hydrogen bond to break down ice into water.
Please watch animation 10.1 (Polarity of
water) on your CD!
There are other intermolecular bonds such as dipole-dipole and induced
dipole-induced dipole. These
intermolecular bonds (Hydrogen bonding, dipole-dipole and induced dipole-induced
dipole) are called van der Waals forces.
Please go to the following web site to
review the special properties of water.
http://www.biology.arizona.edu/biochemistry/tutorials/chemistry/page3.html