(Section 5.2)

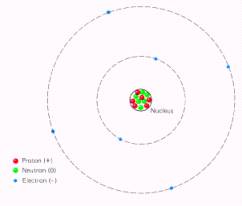
The atom is composed of sub-atomic
particles called protons, neutrons
and electrons. In a
standard view of the atom, the protons and neutrons are in a small area in the
center of the atom called the nucleus. The
electrons circle the nucleus the same way the earth revolves around the sun.
The nucleus is very small compared to the size of the atom.
As an analogy, if the atom where the size of a baseball stadium, then the
nucleus would be smaller than a baseball, perhaps closer to the size of a ping
pong ball. You can imagine that
most of atom is, in fact, empty space.
| |
mass (amu) |
mass (kg) |
relative charge |
charge in coulombs |
| proton | 1 | 1.67 x 10-27 |
+1 |
1.67 x 10-19 |
| neutron | 1 | 1.67 x 10-27 |
0 |
0 |
| electron | 0.00054 | 9.11 x 10-31 |
-1 |
-1.67 x 10-19 |