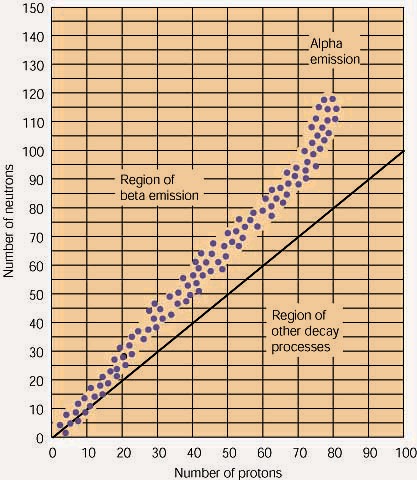
Remember that isotopes above or below the band of stability will decay radioactively.

The trick to doing nuclear equations is that the top numbers must add up and the bottom numbers must add up. When we are looking at atoms, the top number is the mass number and the bottom number is the number of protons. How can we describe some of the types of radiation this way?
Gamma Decay
The gamma ray is not a particle and so cannot be described this way.
Gamma radiation occurs when a nucleus has excess energy.
These are called meta-stable isotopes. Technetium 99 has a meta-stable isotope. A gamma ray can be represented by g.
![]() ®
®
![]() + g
+ g
Beta Decay
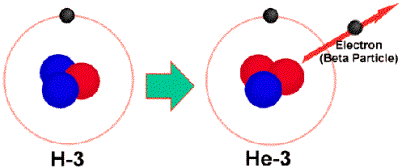
If there are too many neutrons in a nucleus, a neutron to proton conversion
occurs. This releases an electron
or beta particle. This beta
particle can be represented as
![]() . An electron has no protons but it
has an opposite charge so we can make the bottom number be –1.
The electron has essentially no mass so the top number can be 0.
. An electron has no protons but it
has an opposite charge so we can make the bottom number be –1.
The electron has essentially no mass so the top number can be 0.
Carbon-14 undergoes beta decay to the stable nitrogen-14 isotope.
![]() ®
®
![]() +
+
![]()
Look at the top numbers on the left and right of the equation.
14 = 14 + 0
Now look at the bottom.
6 = 7 + (-1)
Positron Emission
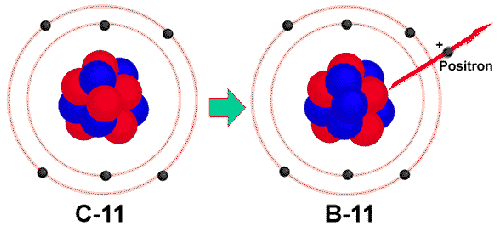
Isotopes on the lower side of the band of stability might want to turn a
proton into a neutron through positron emission.
A positron is essentially a positive electron.
It can be described as
![]() .
.
![]() ®
®
![]() +
+
![]()
Again, Look at the top numbers on the left and right of the equation.
11 = 11 + 0
Now look at the bottom.
6 = 5 + (+1)
Alpha Emission
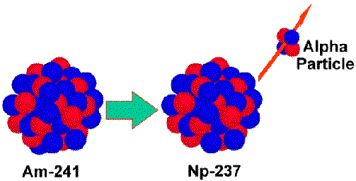
Large isotopes that need to decrease their size tend to decay by alpha
emission. An alpha particle can be
described as a helium nucleus,
![]() , 2 protons and 2 neutrons.
, 2 protons and 2 neutrons.
![]() ®
®
![]() +
+
![]()
Look at the top numbers on the left and right of the equation.
241 = 237 + 4
Now look at the bottom.
95 = 93 + 2
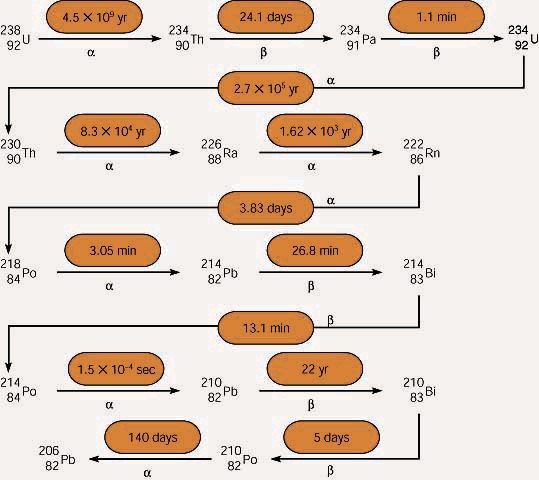
Uranium Decay
As stated earlier, a nuclear decay may not always produce a stable isotope directly. Uranium-238 undergoes 14 decays.