 |
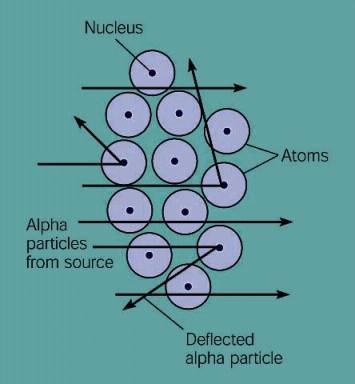
What is the arrangement of particles inside the atom? One would assume that the protons in an atom would move away from each other because positive charges repel each other. Certainly no one would expect them to congregate together. That is exactly what was discovered! Please watch the following movie to see the experiment that showed this to be true.
(That is if you can stand the download time.) http://web.fccj.org/~ksanchez/1025/movies/ruth.movThis one is better: http://micro.magnet.fsu.edu/electromag/java/rutherford/
 |
When Rutherford shot the alpha particle at the gold foil, the last thing he expected was for the particle to bounce back. There should be nothing massive enough to stop the particle. The alpha particle weighs 4 times what a proton weighs. That would be like Tony Bosselli (320 pounds) running at a 10 year old girl (80 pounds) and getting knocked on his butt.
From the observations that most of the alpha particles went straight through and only sometimes were deflected or reflected, Rutherford concluded that all the massive particles were centered in a small area which is called the nucleus. How small is the nucleus compared to the size of the atom?
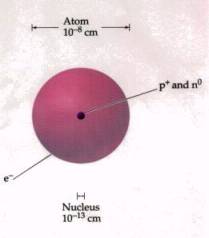
The nucleus is 5 orders of magnitude smaller then the atom.
How big is this difference? If
the atom were the size of a baseball stadium, the nucleus would be smaller than
the baseball.
What holds the protons together in this small area? Whatever the answer, it must be a VERY strong force. In must be stronger than the repulsive forces that drive positive charges apart. The protons are packed very closely together. The density of the nucleus is very large. If a paper clip had the density of a nucleus, it would weigh ten million tons!
You can read about the fundamental forces of nature at the following web page.
http://library.thinkquest.org/3471/nuclear_forces.html