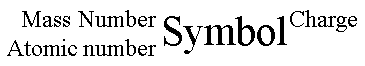
When we examine an individual atom, it is the number of protons that define the element of the atom. The number of protons is the atomic number (Z). All carbons have 6 protons in the nucleus; all chlorines have 17 protons in the nucleus. Most chemists use the periodic table to look up this kind of information. There are many places to find periodic tables on the web. Look at the one at the following site. Use the table to determine the atomic number of tin (Sn). Notice that each element can be represented by a two-letter symbol, the first letter of which is always capitalized, the second letter, if there is one, is not capitalized.
What else is in the nucleus?
Neutrons also reside in the nucleus. In
most stable nuclei, the number of neutrons approximately equals the number of
protons. As the number of protons in the nucleus increases, so does
the number of neutrons. The
neutrons seem to play some kind of role in stabilizing the nucleus.
While a chlorine atom always has 17 protons, it can have any number of
neutrons. Two atoms with the same
number of protons and different number of neutrons are called isotopes.
Two naturally occurring stable isotopes exist for chloride.
One has 18 neutrons and the other has 20 neutrons.
Because protons and neutrons are the only massive particles in the atom,
the mass number of the atom (A) is the protons plus the neutrons.
An ion exists when the protons do not equal the electrons. If there are more electrons than protons than the atom is negatively charged. If there are more protons than electrons than the atom is positively charged. We can describe an individual atom with the symbol in the center, the atomic number in the lower left, mass number in the upper left, and the charge in the upper right.
atomic number = number of protons
mass number = protons + neutrons
charge = protons-electrons
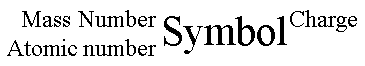
or
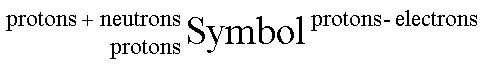
Example:
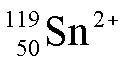 This tin atom has 50 protons, 69
neutrons and 48 electrons.
This tin atom has 50 protons, 69
neutrons and 48 electrons.
This is one of six stable isotopes of tin.
This isotope can also be written tin-119.
When elements are not combined with anything else, they are typically
neutral ( not charged.)
You may want to bookmark this on-line periodic table.
http://periodic.lanl.gov/