When people hear the word “isotopes”, fear of radiation usually comes to
mind. Many elements have more than
one stable isotope. Chlorine for
example has two naturally occurring isotopes:
Chlorine-35 and chlorine-37. Naturally
occurring chlorine is 75 % Chlorine-35
and 25% chlorine-37. It has an
average atomic weight of 35.5 amu. Is
that what is recorded for chlorine on the periodic table?
Some isotopes are not stable. Chlorine has 8 unstable isotopes with half lives that vary from 300,000 years to 286 milliseconds.
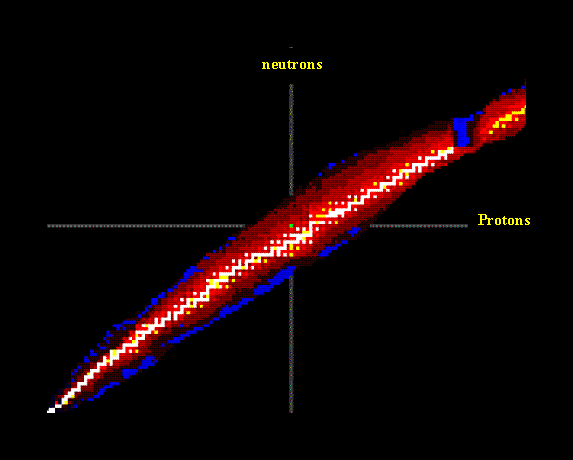
Stable isotopes tend to fall in a band. You can see the band of stability in an applet below.
http://lectureonline.cl.msu.edu/~mmp/kap30/Nuclear/nuc.htm
In this picture, we have plotted protons on the x axis and neutrons on the y-axis. The white dots indicate stable isotopes and the yellow, red and blue dots indicate radioactive isotopes.

Isotopes on the top side of the band tend to decompose by emitting a beta (b) particle. (This lowers the neutron to proton ratio.) Isotopes on the lower side of the band tend to undergo positron emission, electron capture or alpha emission. Each of these raises the neutron to proton ratio. Here is a summary of the decay processes. When an isotope decays, it does not necessarily decay to a stable isotope. uranium-235 decays 14 times before becoming the stable isotope Lead-206.
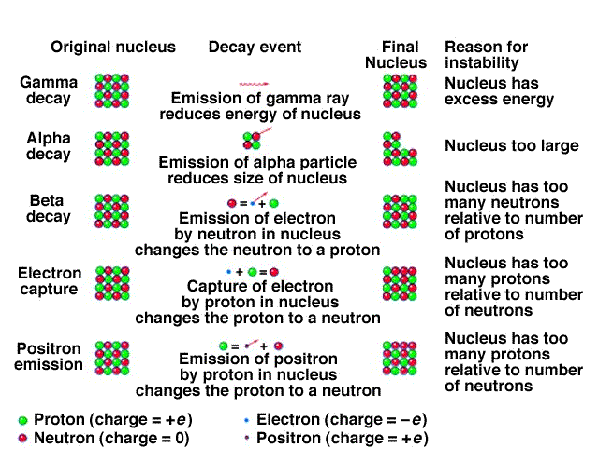
Read about these decay processes at
http://library.thinkquest.org/3471/radiation_types.html