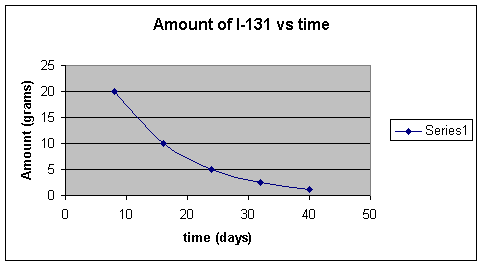
The half-life of a radioisotope is the time it takes for one half of a sample to decay. The decay of an isotope is not linear. Lets look at the decay of radioactive iodine (iodine-131) . Iodine-131 has a half-life of 8 days. Every eight days the amount of radioactive iodine is cut in half. As the amount of radioactive iodine decreases, so does the rate of radioactive emission
|
days |
amount
of I-131 |
|
8 |
20 |
|
16 |
10 |
|
24 |
5 |
|
32 |
2.5 |
|
40 |
1.25 |

Please enjoy this applet
http://lectureonline.cl.msu.edu/~mmp/applist/decay/decay.htm