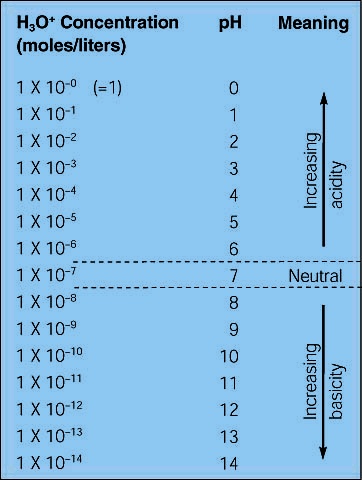
The pH scale
Water auto-ionizes to form a small amount of H+ and OH-. In pure water the H+ concentration equals the OH- concentration. In water the concentrations of H+ and OH- are in a delicate balance, if we increase H+ concentration the OH- will decrease. If we increase OH- concentration the H+ will decrease. Look at the chart below.

In pure water, the H+ concentration is 1x 10-7. As we add an acid the H+ concentration will increase and the pH will decrease. The pH is defined as the negative log of the H+ concentration. As we add base the H+ concentration will decrease and the pH will increase.
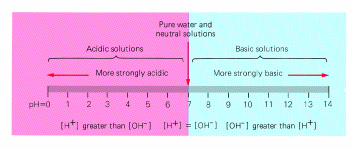
In an acidic solution the H+ concentration is greater than the OH- concentration. In an basic solution the OH- concentration is greater than the H+ concentration.
Please watch animation 10.5 on indicators.