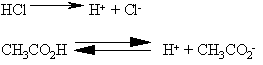
There are more than one set of definitions for acids and bases.
The most common definitions involve a H+ or a proton.
A proton cannot exist by itself in water and so it reacts with water to
form H3O+ (H+
and H2O). Since the
solvent is almost always water, every time you see H+, realize it is
really H3O+. The Arrhenius definitions of acids and bases
are as follows:
An acid is a substance that makes H+ (or H3O+) when dissolved in water. An example of an acid is HCl that splits up in water to form H+ and Cl-. HCl is a strong acid because it completely dissociates. Acetic acid is a weak acid. Only a fraction of acetic acid dissociates into H+ and the acetate ion.
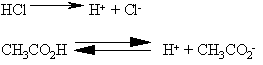
A base is a substance that makes OH- when dissolved in water.
An example of a base is NaOH that splits up in water to form Na+
and OH-. NaOH is a strong base because it completely dissociates.
Ammonia (NH3) is a weak base. Avery small amount of the
ammonia reacts with water to form ammonium hydroxide.
For more information on acids and bases please look at the following web site:
http://www.chem.ubc.ca/courseware/pH/index.html
Acids and bases react to form water. The H+ from the acid reacts with the OH- from the base to form water. HCl reacts with NaOH to form salt and water.
![]()
Acids and bases can react with other things including metals such as aluminum.