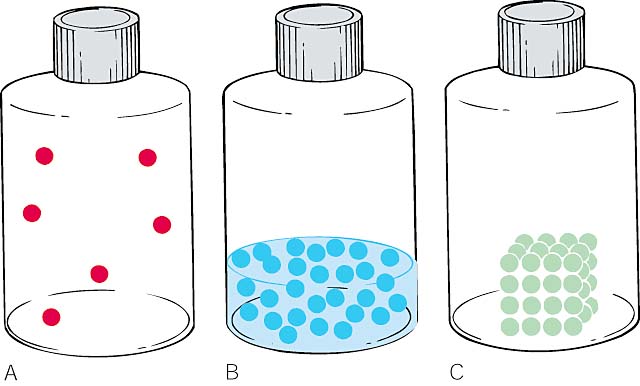
We have already covered these phases of matter. See online section 5.2

(A) In a gas, the molecules are not held together art all so a gas dispenses throughout a container, taking the shape and volume of the container.
(B) In a liquid, the molecules are attracted to each other enough to maintain a volume. The molecules can move past each other so the liquid takes the shape of the container.
(C) In a solid, the molecules are so strongly attracted to each other the molecules cannot move past each other. In a solid, a molecule’s neighbors do not change. A solid retains its own shape and volume.
We will begin by looking at various types of solids. Please read about amorphous solids at http://www.britannica.com/eb/article?eu=119054