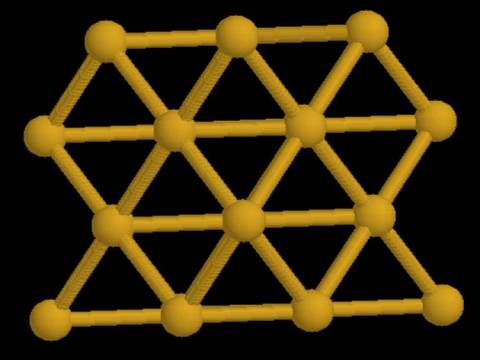
In pure gold, the atoms bond to each other forming a repeating unit similar to the ionic crystals.

(from http://www.tulane.edu/~inorg/Solid%20State%20Structures.html)
Because all the atoms in this crystal are the same, metallic crystals pack efficiently and tend to be fairly dense. The outer shell electrons in a metallic crystal tend to be delocalized, that is they are loosely bound to their nuclei. Metal atoms can be thought of as an array of positive ions immersed in a sea of delocalized valence electrons. The ability of the electrons to move in the crystal accounts for the ability of the metal to conduct electricity.