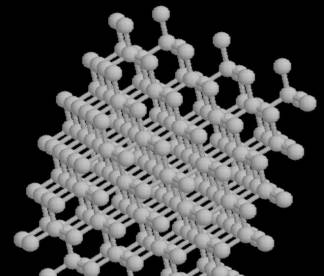
In the diamond, each of the carbons is bonded to four other carbons by covalent bonds. This creates a huge structure. You can look at the whole structure at http://www.recipnet.indiana.edu/common/Minerals/diamond/diamond.htm.

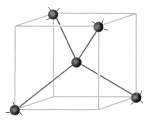
This unit cell builds to a more intricate structure seen below. The exceptional strength is due to the strength of the covalent bonds.