A solution is a mixture in which one substance, called the solute is uniformly distributed in another substance called the solvent. The most common solvent in our everyday life is water. Some examples of solutions are Kool-Aid, cranberry juice, vodka and saline solution. Solutions are typically clear (although they may be colored) and homogeneous (all the same). My cherry Kool Aid is a clear red solution. In this solution the solvent is water and the solutes are sugar, artificial flavor and artificial color. Another interesting property of solutions is that different concentrations of solute can be made. As all of you are aware, you can make very sweet Kool Aid and less sweet Kool Aid.
When the solute particles get big enough so that they refract light, the
solution is called a colloid. Milk is an example of a colloid.
When the particles get big enough to be removed with ordinary filter
paper (coffee filters) then the mixture is a suspension.
Suspensions tend to settle on standing.
Liquid “Kaopectate” and “Milk
of Magnesia” are suspensions
The solubility of a substance is the maximum amount of solute that dissolves
in a given amount of solvent at a given temperature.
A solution that is below the maximum is described as unsaturated and a
solution at the maximum is described as saturated.
A unstable condition can exist where there is more than the maximum
amount of solute in the solvent, this is called supersaturated. (These definitions
of saturated and unsaturated are unrelated to saturated and unsaturated fats.)
Increasing the temperature tends to increase the solubility of a solid in
water and tends to decrease the solubility of a gas in water.
Please read about making rock candy at http://www.beakman.com/rock-candy/rock-candy.html
. When making the candy, you
increase to temperature to increase the solubility of the sugar in the water.
The sugar is saturated at the high temperature.
When the solution cools the solution is supersaturated in sugar and
slowly the excess sugar crystallizes into rock candy leaving a saturated
solution at the lower temperature.
The concentration of a solution is the amount of solute divided by the amount
of solution. The units for these
amounts vary depending on the units of concentration.
It is a measure of the amount of solute per volume of solution.
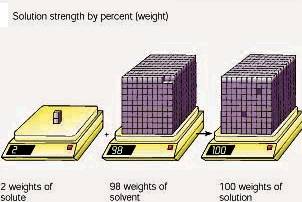
A weight by weight percent is grams of solute per 100 grams of solution. A 2 % sugar solution has 2 grams of sugar per 100 grams of
solution. (This might be considered
parts per hundred.)

Scientists often use parts per million. This is grams of solute per million grams of solution. Concentration is also measured in molarity, which is moles of solute divided by liters of solution. You may remember that moles are proportional to number of molecules. You may calculate these concentrations using the equations below.
![]()
![]()
![]()