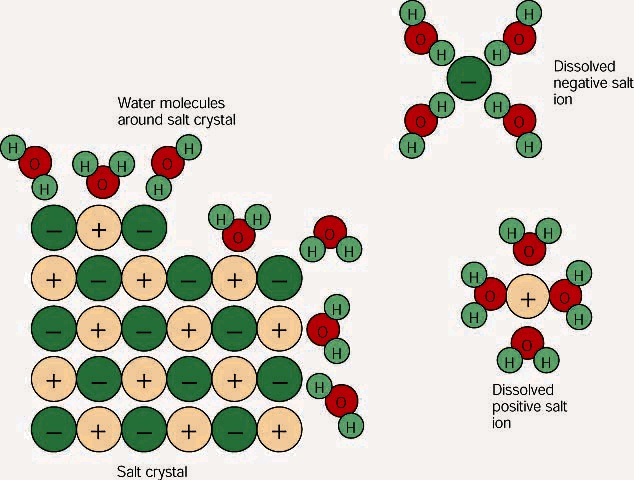
When table salt, sodium chloride, dissolves in water, it dissociates into its
respective cations and anions, Na+ and Cl-.
How does water stabilize the Na+?
It uses the partially negatively charged oxygen side.
One oxygen from the water cannot stabilize the Na+
alone, but several oxygens from different waters can surround the Na +
and their combined partial negative charges can stabilize the Na+.
The chloride is stabilized in the same way by the partially charges
hydrogen side of the waters. Please
examine the picture below.

These free ions in a salt-water solution allow electricity to flow through water. Ionic compounds such as sodium chloride, that dissolve in water and dissociate to form ions, are called electrolytes.
Please Watch animation 10.3 on ionic
solutions.