
All combustion reactions fall into the pattern:
fuel + O2 ->CO2 +
H2O
The coefficients of the balanced equation will change depending on the fuel. The fuel can be almost anything including methane (CH4), propane (C3H8), butane (C4H10), octane (C8H18) or sugar (C6H12O6).
The balanced equation for methane is CH4
+ 2 O2 -> CO2
+
2 H2O

The balanced equation for octane is
2 C8H18
+ 25
O2 ->
16 CO2 + 18
H2O
The combustion of methane or octane is exothermic; it releases energy.
CH4 + 2 O2 -> CO2
+
2 H2O + energy
The energies of the products are
lower than the energiies of the reactants.
The excess energy is released as heat and light.
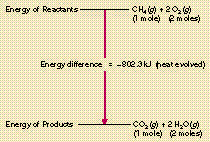
Matter tends to move to lower energy states. A good analogy for a reaction is a ball falling down a hill. Exothermic reactions are more likely to occur. Endothermic reactions absorb energy. In these reactions the products are higher in energy than the reactants. Endothermic reactions are less likely to occur. You seldom see a ball spontaneously going uphill. Endothermic reactions can occur when entropy drives the reaction (pushes the ball up the hill). These reactions are far less common. An exothermic reaction occurs in a chemical hot pack. (Skiers know about hand and foot warmers.) An endothermic reaction occurs in a chemical cold pack.
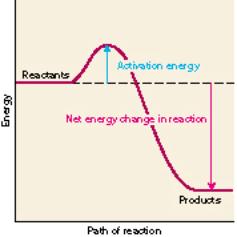
Activation energy
If you mix methane and oxygen together, a reaction does not occur right away. There is a barrier to the reaction. This barrier is due to the fact that to make CO2 and H2O we have to break 4 carbon-hydrogen bonds and some oxygen-oxygen bonds. From this initial energy investment however we get a larger energy payoff when the carbon-oxygen and hydrogen-oxygen bonds are formed.
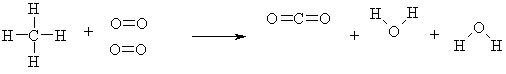
The energy barrier is called the activation energy. The net energy of the reaction is the energy that is released as the methane burns. How do you overcome the initial energy barrier? Typically we light the reactants with a lighter or a spark. Once we start the reaction, the energy released can let other reactions overcome their activation energy and the fire is underway.
(An exothermic reaction)
Please watch the Animation
11.1: Reactions and Energy on your CD