Organic chemists give the following definition to oxidation reactions:
Oxidation: A reaction that increases the number of bonds to oxygen.
Reduction: A reaction that decreases the number of bonds to oxygen.
Organic chemists classify combustions reactions as oxidation reactions
There is another method of classifying oxidation/reduction reaction
Oxidation: When a species loses electrons and becomes more positively charged.
Reduction: When a species gains electrons and becomes more negatively charged.
The following is an oxidation/reduction reaction (redox) reaction.
![]()
Iron is Fe and Fe2O3 is red rust. O2 is oxygen. You might think that water is required for this process but it is not, it merely speeds it up. Notice that the iron is now bound to the oxygen. It has gone from its elemental state with no charge ( Fe0) to its ionic state (Fe3+) Because the iron has lost electrons and become positively charged, it has been oxidized. The oxygen has been reduced. The electrons from the iron went to the oxygen. Every oxidation process has to have a corresponding reduction. We call these redox reactions, the word redox combining reduction and oxidation.
We can write half reactions for this total
reaction:
Oxidation 4 Fe -> 4 Fe3+ + 12 e-
Reduction 3 O2 + 12 e- -> 6 O2-
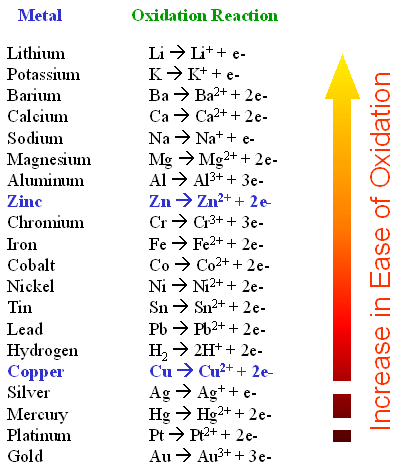
Some metals are more stable in their elemental state than others. Gold, Platinum and silver are hard to oxidize. Other metals such as lithium magnesium and alluminum are easy to oxidize. When they oxidize they change into their ionic state. If you put Zn metal into a solution of Cu2+ and SO42- an interesting thing happens. The zinc goes into solution and the copper begins to plate out as solid copper (Cu0). The equation for this is

The zinc and the copper are solids and the ions are in solution.
Notice that the sulfate (SO42-) is not
changing
during the reaction. We call these
ions spectator ions. Why does this
reaction occur? Copper is more
stable than zinc. Copper is below
zinc on the activity series.
The activity series.
The redox half-reactions for the above reaction would be:
Oxidation: Zn(s)
->Zn2+(aq) + 2e-
Reduction: Cu2+(aq)
+ 2e- ->Cu(s)
The oxidation of a Zinc atom releases 2 electrons
The reduction of a Copper ion is achieved by the acceptance of 2 electrons
Thus, there would appear to be a
movement, or
flow, of electrons from the Zinc metal to the Copper ions
We can capture the power energy produced in this reaction in a battery. The battery can use this flow of electrons to do work. Please read about voltaic cells at
http://wine1.sb.fsu.edu/chm1046/notes/Electro/Voltaic/Voltaic.htm
or
http://sciborg.uwaterloo.ca/~cchieh/cact/c123/battery.html
The lead-acid storage battery uses lead 0 (elemental) and lead 4+.
Oxidation: Pb(s) ->
Pb2+(aq) + 2e-
The lead gives away two electrons
through the
wire and becomes Pb2+which combines with SO42-
to become lead(II)sulfate.
Reduction: Pb4+(s)
+ 2e- ->
Pb2+(aq)
The lead
here starts as
Pb4+ as it combines with 2 O2-
. It
can take the two electrons from the wire and become Pb2+which
combines with SO42- to become lead(II)sulfate.
Again, as
the electrons
pass through the wire they create a voltage which can be used to do
work.
