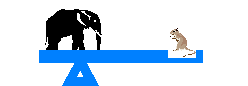
Equilibrium is defined as an exact balancing of two processes that are opposite of each other. Think of a tetter totter.
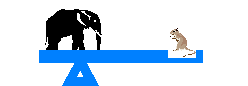
Notice that the weights of the two animals is not the same but by shifting their position they can achieve equilibrium.
Chemical equilibrium is defined as a dynamic state where the concentration of all reactants remains constant. They may not be equal but they are not changing.
In a chemical reaction, a double arrow indicates an equilibrium situation. Reactants are on the left and products are on the right.
![]()
We have made an implicit
assumption… that reactions react to completely transform reactants to
products.
Equilibrium is..
1.
A state in which the concentrations of reactant and product are
no longer
changing, for a reversible reaction.
2.
A state in which the rate of the forward reaction is equal to
the rate of
the reverse reaction; reactant is being consumed to form product at the
same
rate at which product is being degraded to form reactant.
3.
Individual molecules are constantly being converted from one
form to
another, but no overall change in the concentrations of any species is
observed.
What do we mean by dynamic?

An example of an equilibrium reaction is
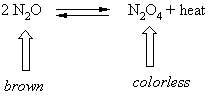
By dynamic we mean that N2O is constantly going to N2O4 and N2O4 is constantly going to N2O. They are just balancing each other out. This reaction is exothermic so heat can be considered a product.
We can perturb a system at equilibrium. (Remember our tetter totter. We can add some cheese to the mouse side). How will this affect the relative ratios? Le Châtelier's Principle says the following:
"A
system at equilibrium, when stressed, will shift to
offset the stress”
This
means if we add reactant, equilibrium goes right, away from
the reactant.
If
we add product, equilibrium goes left, away from the product.
If
we remove product, equilibrium goes right, making product.
If we remove reactant, equilibrium goes left, making reactant.
Please watch Animation
11.3: Shifting Equilibrium
Notice that in the second video we are watching this reaction
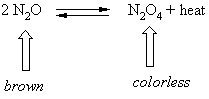
By cooling we are removing heat and the equilibrium shifts right and the color gets lighter because we are converting N2O to N2O4.
By heating we are adding heat and the equilibrium shifts left and the color gets darker because we are converting N2O4 to N2O.
Lets look at the first reaction
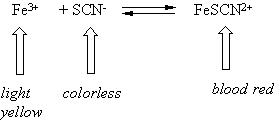
By adding iron (Fe3+) or thiocyanate (SCN-) we shift the equilibrium to the right making more blood red iron/thiocyanate complex and the solution gets darker.