 |
Temperature (T): Temperature
is related to the kinetic energy of the gas and is measured in Kelvin (K).
Since the kinetic energy is ½ mv2, the same molecule will
increase in velocity as temperature increases.
Amount of gas (n): Typically
measured in moles.
Volume: In a closed
system, the volume of the gas is the same as the volume of the container.
Typically measured in liters.
Pressure in a closed system: Typically measured in pascals (Pa) or atmospheres (atm). 133 Pa = 1 atm. Pressure is force/area. The force of the gas comes from the molecules hitting the sidewall of the container. The area is the areas of the wall of the container.

Pressure in an open system (outdoors): Also measured in pascals (Pa)
or atmospheres (atm). We feel
the pressure of the air around us. What is the pressure of the atmosphere on
your book sitting on your desk? Pressure
is still force/area. The area is the areas of the top of the book.
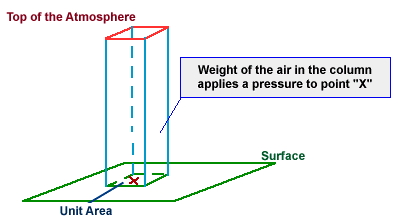
Imagine a column of gas
extending from your book to the top of the statosphere.
The force comes from the weight of that gas pushing down. (F=mg where g=
9.8m/s2).
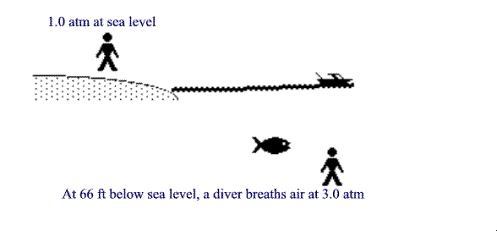
Pressure under water.
Water weighs considerably more than air does, so it can exert much more
pressure. It takes only 33 feet of sea water to weigh the same as all the air
from sea level to the top of the stratosphere. . This means that at a depth of
33 feet deep in the ocean, there is a total pressure of 2
ATMs of pressure. One ATM from the water, + one ATM from the water.
Every additional 33 feet of sea water, will add another ATM. At 66 feet
we have two ATMs of water pressure plus our 1 ATM of air pressure for an
absolute pressure of 3 ATM.
Scuba divers use a regulator to breath air at those pressures. This concentration can sometimes cause problems with their blood chemistry.