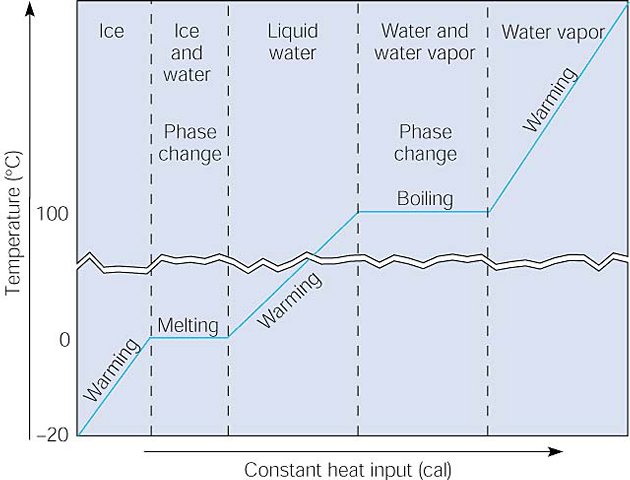

This graph shows three warming sequences and two phase changes with a
constant input of heat. The ice warms to the melting point, and then absorbs
heat during the phase change, as the temperature remains constant. When all the
ice has melted, the now liquid water warms to the boiling point, where the
temperature again remains constant as heat is absorbed during the second phase
change from liquid to gas. After all the liquid has changed to gas, continued
warming increases the temperature of the water vapor.