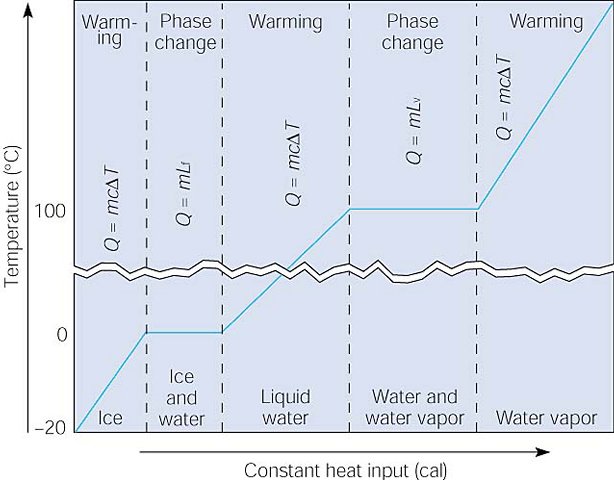

Compare this graph to the one in on the previous page. This graph shows the
relationships between the quantity of heat absorbed during warming and phase
changes as water is warmed from ice at -20oC to water vapor at some
temperature above 100 oC. Note that the specific heat for ice, liquid
water, and water vapor (steam) have different values.
It takes a lot of energy to convert water (liquid) in to steam (gas).
The heat of vaporization of water (Lv on
the chart) is 2260 kJ/kg.
Sample problem:
How much energy is required to vaporize 700 g of water?
Answer:
Heat
energy = mass times the heat of vaporization or
Q=m x Lv
I
have to make my units match up so I will convert 70 g to 0.700 kg.
Q=m x Lv = 0.700kg x 2260
kJ/kg = 1582 kJ