Temperature is a measure of the average kinetic energy of a molecule or atom. It is related to ½ mv2 where m is the mass of the particle and v is the velocity. In gases it is easy to visualize the velocity of the particles. In solids, since the molecules do not change neighbors, it is hard to visualize the velocity of the particles. In solids, the velocity is related to how the particles are vibrating in place.
Lets examine the effects of temperature and mass. Start up the Molecules in motion applet.
Increase the mass of the red particles. When you increase the size of the molecules the velocities slow down. That is because at the same temperature gases have the same average kinetic energy. Think of me (160 pounds) and Tony Boselli (320 pounds) . I will have to be moving much faster to have the same energy as Tony.
Decrease the temperature of the red particles. When you decrease temperature you decrease kinetic energy and you decrease velocity.
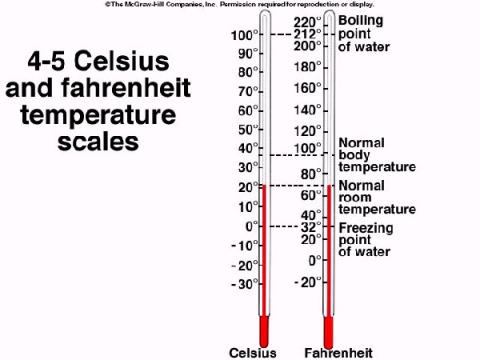
Temperature Scales. We are familiar with two temperature scales, Fahrenheit and Celsius (formerly called Centigrade). The boiling point of water is 212°F and 100°C and the freezing point of water is 32 °F and 0°C. These are both scales arbitrarily designed by people. We can see that the temperature value of a degree Fahrenheit is less than a degree Celsius because the difference between the boiling and freezing point of water is divided up into 180 °F and only 100 °C. We also see that they have different relative starting points. The relationship between these scales is defined by the following equations:
![]()
Sample Problem: What is the
Celsius temperature for 98.6 °F?
Answer: ![]()
The Kelvin Scale.
In his work on gasses, Lord Kelvin found it convenient to define a new temperature scale. In this scale, zero corresponds to zero kinetic energy. He based the scale on the Celsius scale and temperatures in this scale are designated K. We can imagine that this is the lowest temperature possible because after molecular motion stops, molecules cannot move any slower.
This point then is called absolute zero and is 0 K, which is equal to -273°C.
At this point, we have been unable to cool matter to 0 K, although we have come
very close.
|
|
Fahrenheit | Celsius | Kelvin |
|
Boiling point of water |
212 |
100 |
373 |
|
A warm day |
86 |
30 |
303 |
|
Freezing point of water |
32 |
0 |
273 |