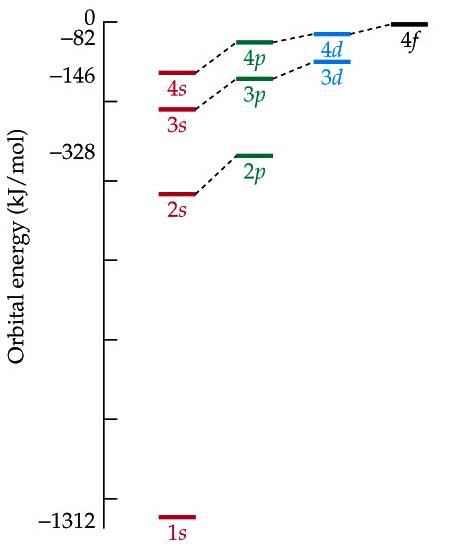
In an atom with more than one electron, s and p orbitals of the same shell do not have the same energy. The 2s and 2p orbitals are all part of the second shell of the Bohr atom. Using the Schrödinger equation increases the complexity of our model of the electron. Hopefully we will be able to use mostly the Bohr model as we examine the complexities of chemical compounds.
