Atomic Spectra and the Bohr Model
We have already discussed what color is.
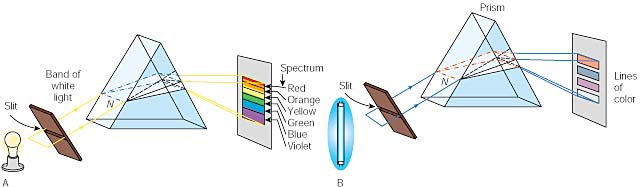 Light
from incandescent solids, liquids, or dense gases, produces a continuous
spectrum. If we pass this light
through a thin slit and then through a prism, the prism will separate the colors
into a spectrum from red (lowest energy) to violet (highest energy).
This does not surprise us as we remember that white light is a
combination of many wavelengths. (Black
is the absence of light.)
Light
from incandescent solids, liquids, or dense gases, produces a continuous
spectrum. If we pass this light
through a thin slit and then through a prism, the prism will separate the colors
into a spectrum from red (lowest energy) to violet (highest energy).
This does not surprise us as we remember that white light is a
combination of many wavelengths. (Black
is the absence of light.)
An interesting thing happens when we use light just from one element, such as
hydrogen. Light from this gas
produces a line spectrum that contains only certain frequencies.
Neon lamps are similar to these lamps.
The lamps get there energy from the electrical potential energy of the
wall socket and convert that to radiant or light energy.
Bohr came up with an interesting
explanation of this phenomenon. He
stated that the electrons orbit the nucleus the way the earth orbits the sun.
An electron in an atom can only exist in certain orbits around the
nucleus. I have drawn 4 orbits but there are more.
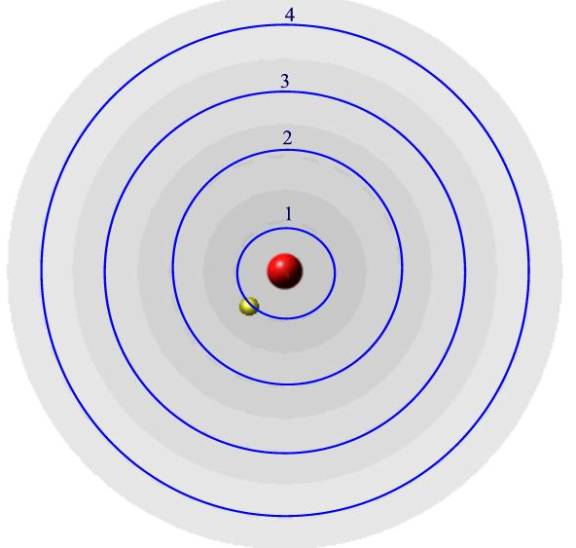
What Bohr said was that the electron could only exist in these blue orbits,
not in between. Each orbit has a
definite energy with the inner orbit being the lowest in energy.
The energy of the orbits increases as you get further from the nucleus.
By saying that the electron can only have specific energies, we can say
that the energies of the electrons are quantized. (The beginning of quantum mechanics.)
The electron is normally in the lowest orbit (orbit number 1) which
is also called the ground state. In
this experiment it is excited into a higher orbit by the electrical energy (say
to orbit number 4.) As is comes
back to a more stable orbit it can release the energy as light of a specific
wavelength.
The transitions to 1 (2 to 1, 3 to 1, 4 to 1 etc) are all in the UV region
and we cannot see them. The
transitions to 2 (3 to 2, 4 to 2, 5 to 2 etc) are in the visible region.
This explains why hydrogen absorbs only specific wavelengths of light and
emits only certain wavelengths. It
is because the electrons can only be in these distinct orbitals.
Look at the following applet and start with hydrogen.
When a broad visible spectrum of light shines on hydrogen, notice it only
removes 4 wavelengths of light. The
red absorption line comes from an electron absorbing that wavelength and going
from orbit 2 to orbit 3.
While you are there, notice that each of the elements has a different pattern
of absorption. Do you think that we
can use this to analyze what some matter is made of?
YES!
http://javalab.uoregon.edu/dcaley/elements/Elements.html
Now look at emission. Each time an electron males a "quantum leap,"
moving from a higher energy orbit to a lower energy orbit, it emits a photon of
a specific frequency and energy value. Hydrogen emits only 4 wavelengths.
The red line comes an electron going from 3 to 2. Here is a chart that
shows the various energies and the corresponding transitions.
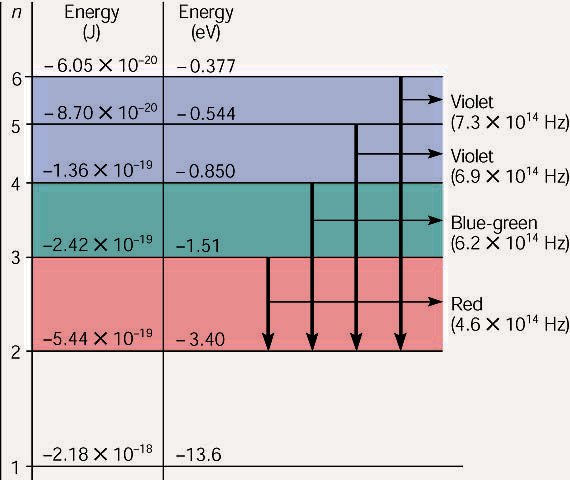
An energy level diagram for a hydrogen atom, not
drawn to scale. The energy levels (n) are listed on the left side, followed by
the energies of each level in J and eV. The color and frequency of the visible
light photons emitted are listed on the right side, with the arrow showing the
orbit moved from and to.
Please look on your CD for animations regarding absorbsion and emission or go
to the following two web sites. (They are the same movies as on your CD, animations 8.2 and
8.3)
Absorption movie:
http://webphysics.ph.msstate.edu/jc/library/27-1/index.html
Emission movie: http://webphysics.ph.msstate.edu/jc/library/27-6/index.html
 Light
from incandescent solids, liquids, or dense gases, produces a continuous
spectrum. If we pass this light
through a thin slit and then through a prism, the prism will separate the colors
into a spectrum from red (lowest energy) to violet (highest energy).
This does not surprise us as we remember that white light is a
combination of many wavelengths. (Black
is the absence of light.)
Light
from incandescent solids, liquids, or dense gases, produces a continuous
spectrum. If we pass this light
through a thin slit and then through a prism, the prism will separate the colors
into a spectrum from red (lowest energy) to violet (highest energy).
This does not surprise us as we remember that white light is a
combination of many wavelengths. (Black
is the absence of light.)
