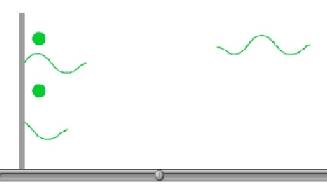
Please watch animation 8.1: The Photoelectric Effect

The photoelectric effect is the observation that under certain conditions,
light striking a metal surface can cause electrons to be ejected.
In the animation, the light increases in energy as it goes from red, to
orange to yellow to green and then blue. Notice
how only the higher energy green and blue can cause electrons to be ejected.
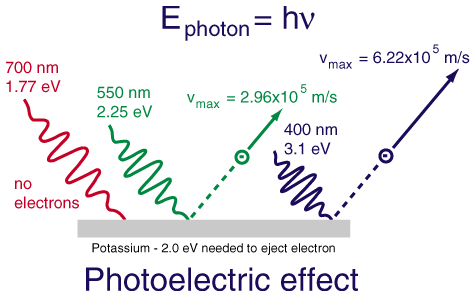
Einstein's definition of photons comes from this photoelectric effect and the following experiment. A fairly high voltage difference is created between two pieces of metal.
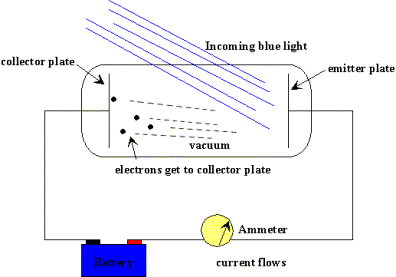
The observations are as follows: Initially with the plates in the dark, no electrons pass between the plates and no current flows. As we shine light on the plates, going from low energy red to high energy blue, suddenly current begins to flow. How?
Einstein explained the result be describing the light waves as packets of energy. He called the packets of energy photons. Each photon has a specific energy, the shorter the wavelength, the higher the energy. When a high-energy photon hits the plate (such as a green or blue), the photon has enough energy to knock out an electron and then the electron, being negative, will fly to the positive side. At this point, current flows.
The lower energy photons are unable to know out the electrons and no energy can flow.
The exciting thing to note is the treatment of energy as a particle or a packet, it is consistent with the fact energies are not additive. If we can bump electrons with light at 600 tera-hertz , we canít just use 300 tera-hertz for twice the time and still expect to bump electrons.
What if the photon has greater than the threshold energy required to pop out an electron? The extra energy just goes into the kinetic energy of the electron. This relationship can be described by the following equation.
hf = w + KE
where hf is the energy of the photon.
w is the energy required to bump an electron.
KE is the kinetic energy of the electron.
If the photon has less energy than w, no electron is emitted.