The first quantum number is the principle quantum number, n. This quantum number can be 1, 2, 3... It is most closely related to the shell or orbits of the Bohr model.

(A) A contour representation of an s orbital. (B) A
contour representation of a p orbital.
The second quantum number is the orbital quantum number or the angular momentum quantum number, l. This represents the type of orbital. If l = 1 then the electron is in a s type orbital. If l = 1 then the electron is in a p type orbital. “s” orbitals are sphere shapes while “p” orbitals are dumbbell shaped. “p”-type orbitals come in groups of three, one is aligned along the x axis, one along the y axis and one along the z axis. We call these px, py and pz.
There are also d and f orbitals. To look at pictures of these orbitals, go to this web site.
http://www.albany.net/~cprimus/self/orbtable.htm
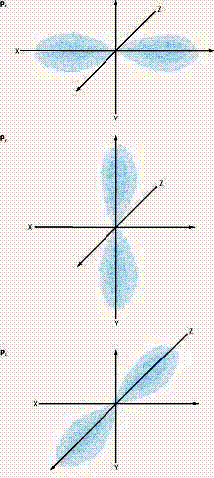
There are three possible orientations of the p orbital, and these are called px, py and pz. The third quantum number, the magnetic quantum number, ml, describes which of the p orbitals, x y or z, the electron is in.
The final quantum number is spin, ms. Electrons behave in some respects as if they were tiny charges spheres spinning around an axis. This spin (blue arrow) gives rise to a tiny magnetic field (green arrow). The values for spin are +1/2 or –1/2.
The Pauli exclusion principle states that an electron in a atom cam be
described by a unique set of quantum numbers.
This means that in an atom with more than one electron, no
two electrons can have the same set of 4 quantum numbers.
