Substances react to form other substances in chemical reactions. We often identify a chemical reaction because we observe a temperature change, a color change (not just a dilution but a change from say blue to red,) a solid forming or a gas forming. Sometimes chemical reactions occur and we are unable to see it with ours eyes.
When we describe chemical reactions, we show the reactants on the left and the products on the right separated by an arrow.
![]()
Chemical reactions do not create enough energy cause a measurable amount of change in mass through the equation E=mc2 so mass must remain constant. We are not changing the nucleus so the number of each of elements must remain constant.
In the reaction of hydrogen and oxygen to make water, 2 hydrogens are required to react with one oxygen so that the equation is balanced.
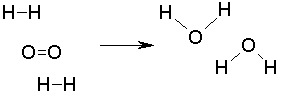
The equation for this reaction is
2H2 + O2 -> 2 H2O
Please go to the following site to work on balancing equations:
http://www.wfu.edu/~ylwong/balanceeq/balanceq.html
Please also do methane, ethane and propane on the Interactive
exercises on balancing equations.