 |

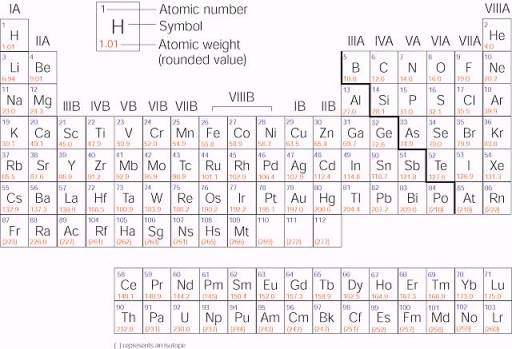
Elements are arranged by reactivity in the periodic table. Elements with similar reactivity are put into the same column or group. Some of these groups have special names. The elements in group IA are called the alkali metals. The elements in group IIA are called the alkaline earth metals. The elements in group VIIA are called the halogens and the elements in group VIIIA are called the noble gases or the inert gases. The metals in group IB (copper, silver and gold) are sometimes called the coinage metals. The columns with B (IB through VIIIB) are called the transition elements. The columns with A (IA through VIIIA) are called the main group elements.
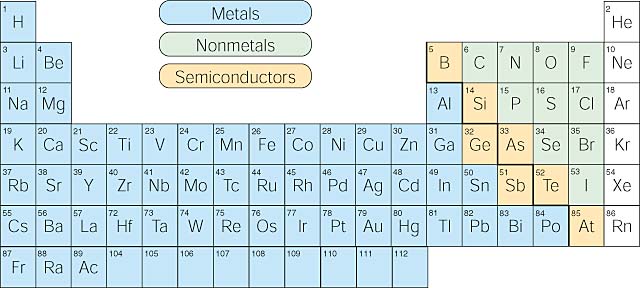
The elements can also be divided into two main groups, the metals and the non-metals. Metals are typically have a metallic sheen (shiny) are malleable (bendable) and conduct electricity. Nonmetals typically do not show these properties. There are some elements that show some, but not all, of the metallic properties. These elements are called metalloids and are labeled here are semi-conductors.
Electrons are the “glue” that hold atoms together in compounds. It is the outer shell electrons that form these bonds between atoms. The first two quantum numbers n (the shell) and l (the subshell) are both important in understanding bonding. In this class we focus on the shell. The shells correspond to the orbits of the Bohr model. (See lecture 10.3)
The first shell is the smallest so it can only hold a maximum of 2 electrons. The second shell can only hold a maximum of 8 electrons. The third shell can only hold a maximum of 18 electrons but is particularly stable at 8 electrons.
Because it is the outer shells that react, we are most interested in the outer shell electrons. We can represent the number of electrons in the outer shell with dots. The outer shell are given the name valence electrons. Officially, the valence electrons are the electrons in the outer shell of the uncharged atom. Chlorine has 7 electrons in its outer shell and so can represent it as a “Cl” with seven dots around it.
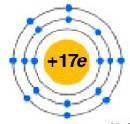 becomes
becomes
![]() . Notice how 2 electrons in the
first shell and the 8 electrons in the second are inner shell electrons and are
not written with dots. Here is a
chart of the main group elements and their Lewis dot symbols.
. Notice how 2 electrons in the
first shell and the 8 electrons in the second are inner shell electrons and are
not written with dots. Here is a
chart of the main group elements and their Lewis dot symbols.

Notice that for the main group elements, the number of valence electrons is equal to the group number.