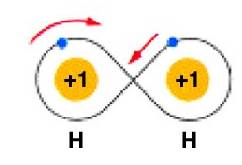

An individual hydrogen atom has 1 electron. The closest noble gas, helium, has 2 electrons. Two hydrogens come together to share their electrons with each other. Each hydrogen now feels like it has two total electrons. Hydrogen exists in its elemental state as H2, a molecule made of two hydrogens. The Lewis structure for hydrogen can be represented as shown in figure B but often a shared pair of electrons is shown as a dash, as shown in figure C.
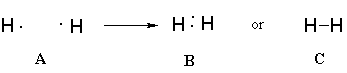
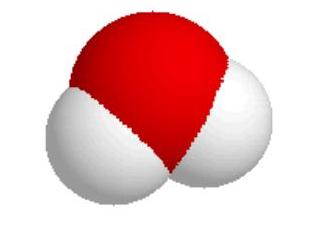
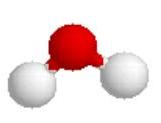
Water is another covalent molecule. Notice that both oxygen and hydrogen are non-metals. The formula for water is H2O, it contains 2 hydrogens and 1 oxygen. The oxygen has 6 electrons in itís valence shell and the hydrogen each has 1. When the hydrogen and oxygen each share 1 electron with each other, then all the atoms feel like they have a complete set. Elements in the first period, like hydrogen, try to get 2 electrons in their outer shell. Elements in the second period and above, like oxygen, try to get 8 electrons in their outer shell.

The Lewis structure describes what is bonded to what but does not really
describe what the molecule looks like. Two
descriptions are shown below. The
one on the left is a space-filling model and the one on the right is a ball and
stick description.
Like hydrogen, oxygen exists in its elemental state as a diatomic molecule. Each oxygen has 6 electrons and so need to share to more and so makes two bonds with the other oxygen. This is called a double bond.

Nitrogen forms a triple bond. The nitrogens are sharing 6 electrons, 2 in each bond.
![]()
I am afraid that the R. Logan web site below is no longer valid. Enjoy his new site if you want anyway.
http://members.aol.com/profchm/covalent.html