For an ionic compound to be stable, the positive charges have to equal the
negative charges. In NaCl, sodium
(Na) is +1 and the chloride is –1. In MgO the magnesium is +2 and the oxygen (O) is –2 and so
again the charges cancel each other out. Remember
the charges come from the chart above.
Can we combine sodium (Na+) with oxygen (O2-)? Yes! To make a stable compound we need 2 sodiums for every one oxygen.
![]()
There is rule for finding the correct formula. In every ionic formula the cation is written first and the anion written second. In the formula, the charge on one becomes the subscripts of the other.
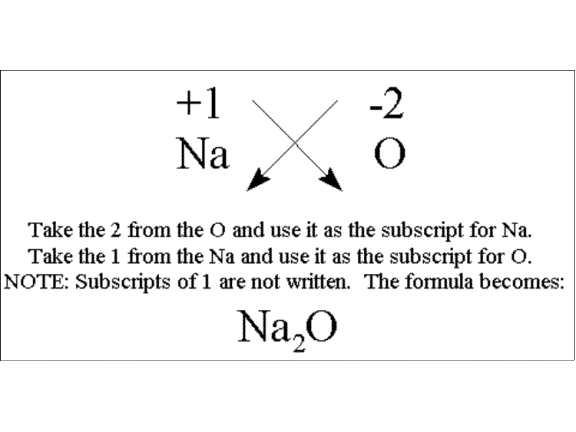
The only corollary to this is that if you can divide both subscripts by an integer greater than one, you must do so. For example, Mg2+ and O2- do not form Mg2O2 but MgO.
This works for many compounds:
|
Ba+2 Cl- |
becomes |
BaCl2 |
|
Al+3
O-2 |
becomes |
Al2O3 |
|
Ca+2
N-3 |
becomes |
Ca3N2 |
|
K+1
P-3 |
becomes |
K3P |
Please go to this site for more information on writing formulas. http://web.fccj.org/~smilczan/psc/bond.html